Feature extraction using CellProfiler
In this tutorial, we show how to use Squidpy with functions from CellProfiler pipelines for image processing and feature extraction.
We’ll go through the following steps:
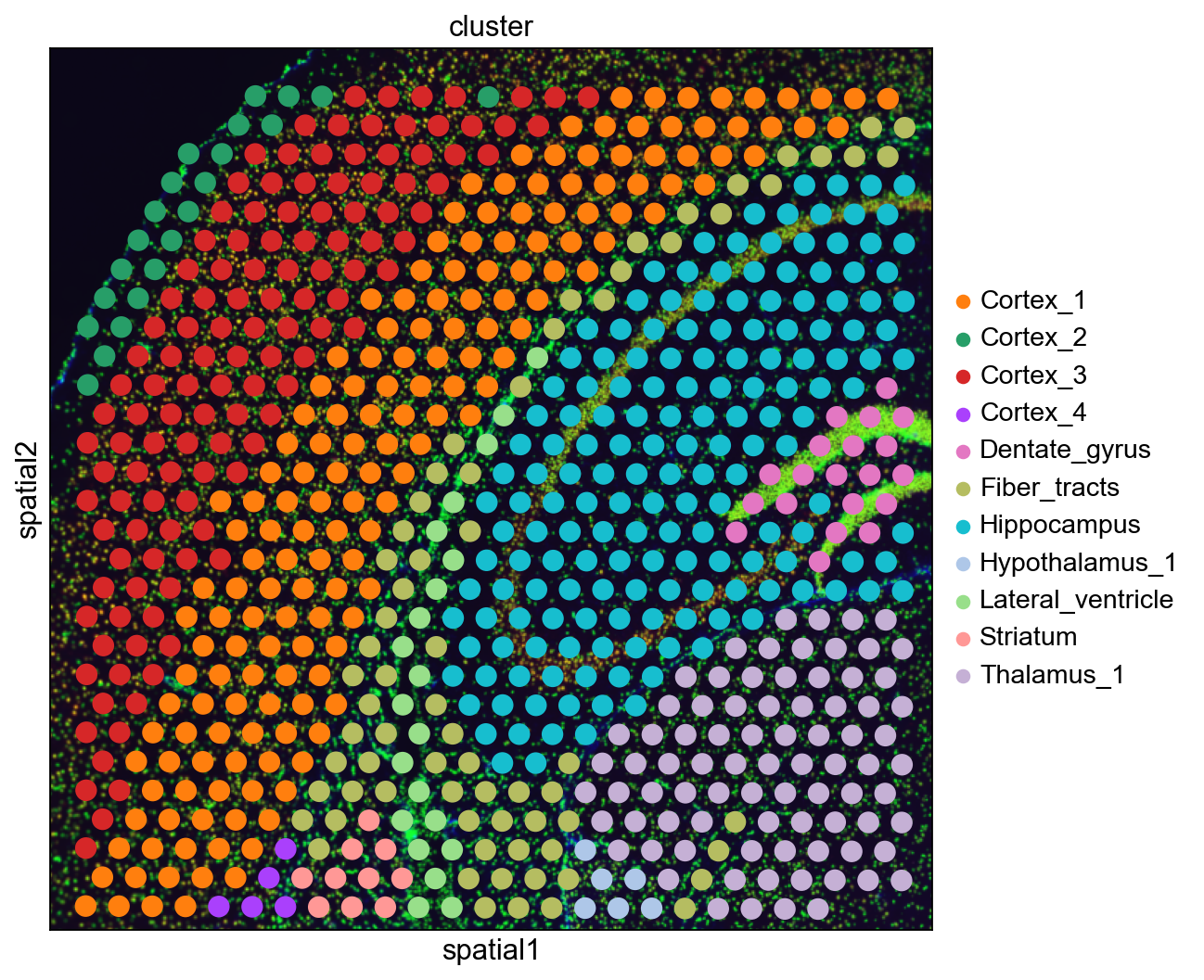
Load Visium fluorescence data.
Segment cells in Squidpy.
Calculate CellProfiler’s granularity features for image crops of Visium spots.
Compute clustering on the image features in Squidpy.
CellProfiler is typically used via its GUI interface to build image processing pipelines. First, download and install CellProfiler from the download page. Check the issues on CellProfiler Github in case of installation problems (can be tricky).
Note: In the future, CellProfiler functions will also be accessible via Python directly (according to the announcements of the CellProfiler team). Since this is not yet publicly well documented, we’ll restrict this tutorial to the laborious way of saving intermediate files to bridge Squidpy and CellProfiler. For information on how to use the cellprofiler-core package for Python integration, the following link might be helpful.
Import packages & data
import itertools
from pathlib import Path
from PIL import Image
import numpy as np
import pandas as pd
import matplotlib.pyplot as plt
import anndata as ad
import scanpy as sc
import squidpy as sq
sc.logging.print_header()
print(f"squidpy=={sq.__version__}")
sc.set_figure_params(facecolor="white", figsize=(8, 8))
# load the pre-processed dataset
img = sq.datasets.visium_fluo_image_crop()
adata = sq.datasets.visium_fluo_adata_crop()
scanpy==1.9.1 anndata==0.8.0 umap==0.5.3 numpy==1.21.6 scipy==1.8.0 pandas==1.4.2 scikit-learn==1.0.2 statsmodels==0.13.2 python-igraph==0.9.9 pynndescent==0.5.6
squidpy==1.2.2
sq.pl.spatial_scatter(adata, color="cluster")
Setup files
Create some arbitrary empty base directory BASE_DIR and a folder imgs_dir = BASE_DIR + "tmp_imgs" in it.
BASE_DIR = "./squidpy_tutorial_cellprofiler/"
imgs_dir = BASE_DIR + "tmp_imgs/"
Path(imgs_dir).mkdir(parents=True, exist_ok=True)
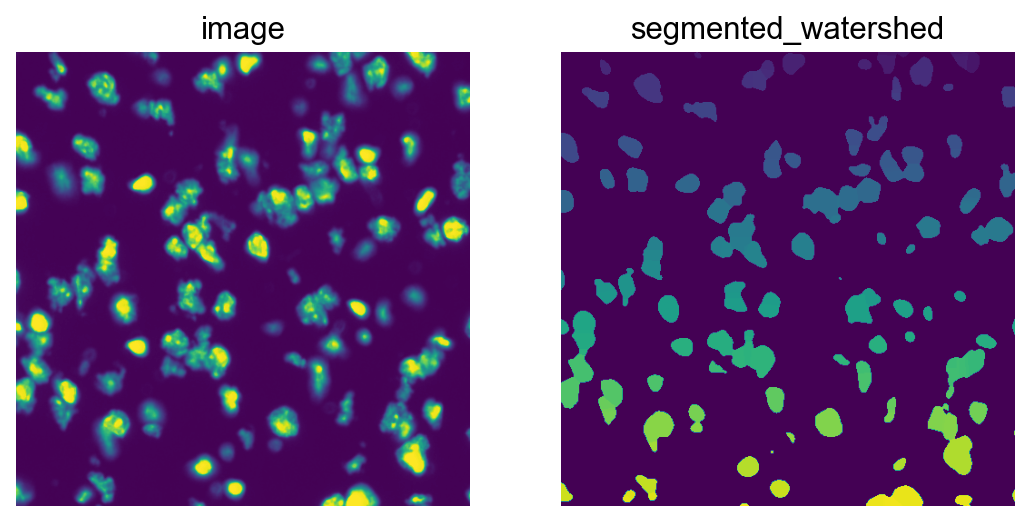
Image segmentation
sq.im.process(
img=img,
layer="image",
method="smooth",
sigma=[2, 2, 0, 0],
)
sq.im.segment(
img=img, layer="image_smooth", method="watershed", channel_ids=0, chunks=1000
)
# plot the resulting segmentation
fig, ax = plt.subplots(1, 2)
img_crop = img.crop_corner(2000, 2000, size=500)
img_crop.show(layer="image", channel=0, ax=ax[0])
img_crop.show(
layer="segmented_watershed",
channel=0,
ax=ax[1],
)
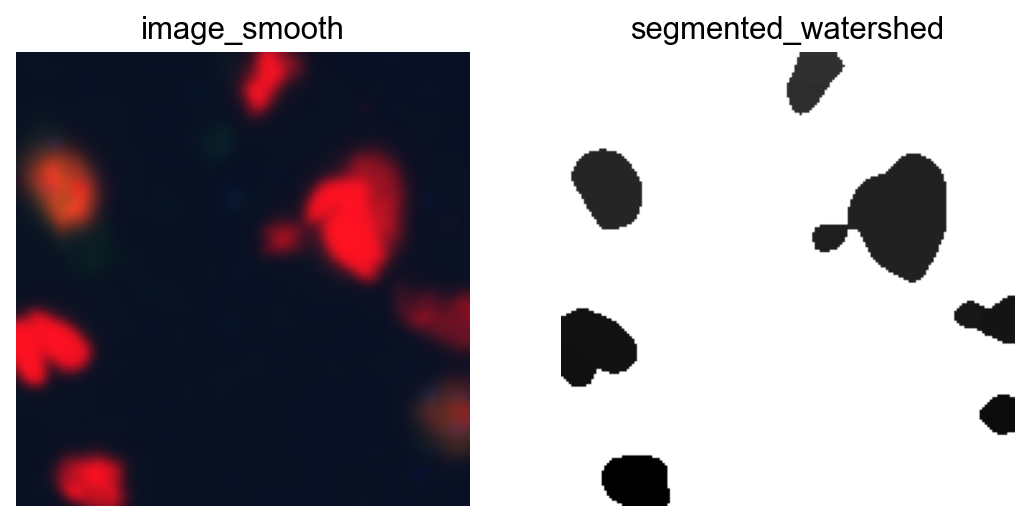
Save image crops and segmentations
For each Visium spot we generate crops:
for crop, obs in itertools.islice(
img.generate_spot_crops(
adata, obs_names=adata.obs_names, return_obs=True, as_array=False
),
2,
):
fig, axes = plt.subplots(1, 2)
crop.show("image_smooth", ax=axes[0])
crop.show("segmented_watershed", ax=axes[1], cmap="Greys")
plt.show()
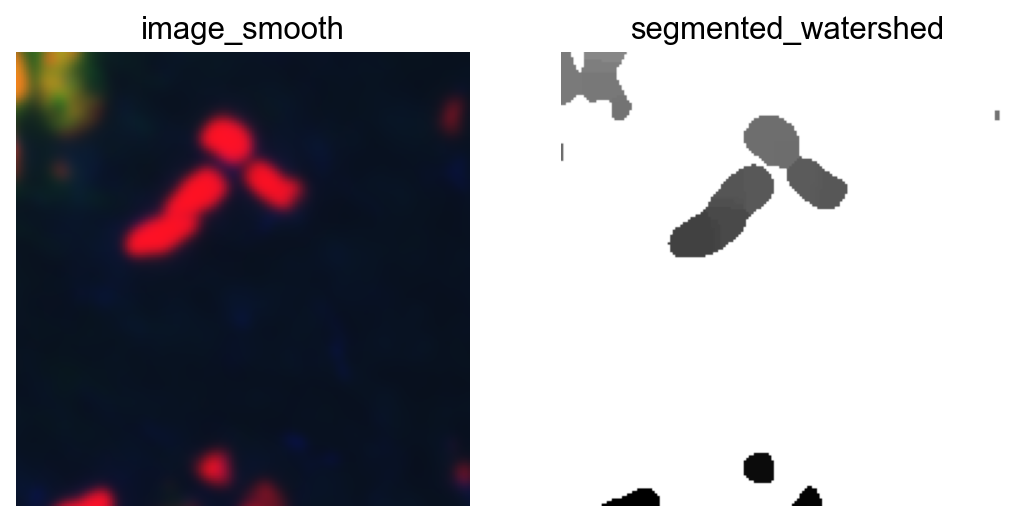
Save the fluorescence image and the segmentations of each crop.
for crop, obs in img.generate_spot_crops(
adata, obs_names=adata.obs_names, return_obs=True, as_array=False
):
Image.fromarray(
(np.array(crop["image_smooth"]) * 255).squeeze().astype(np.uint8)
).save(imgs_dir + f"image_{obs}.png")
Image.fromarray((np.array(crop["segmented_watershed"][:, :, 0])).squeeze()).save(
imgs_dir + f"segments_{obs}.tif"
)
CellProfiler Pipeline: Calculate Image Features
1. Open the CellProfiler GUI App. A new project should open automatically.
2. Save the project in BASE_DIR.
3. CP-Pipeline Images: Drag and Drop the folder imgs_dir into CellProfiler.
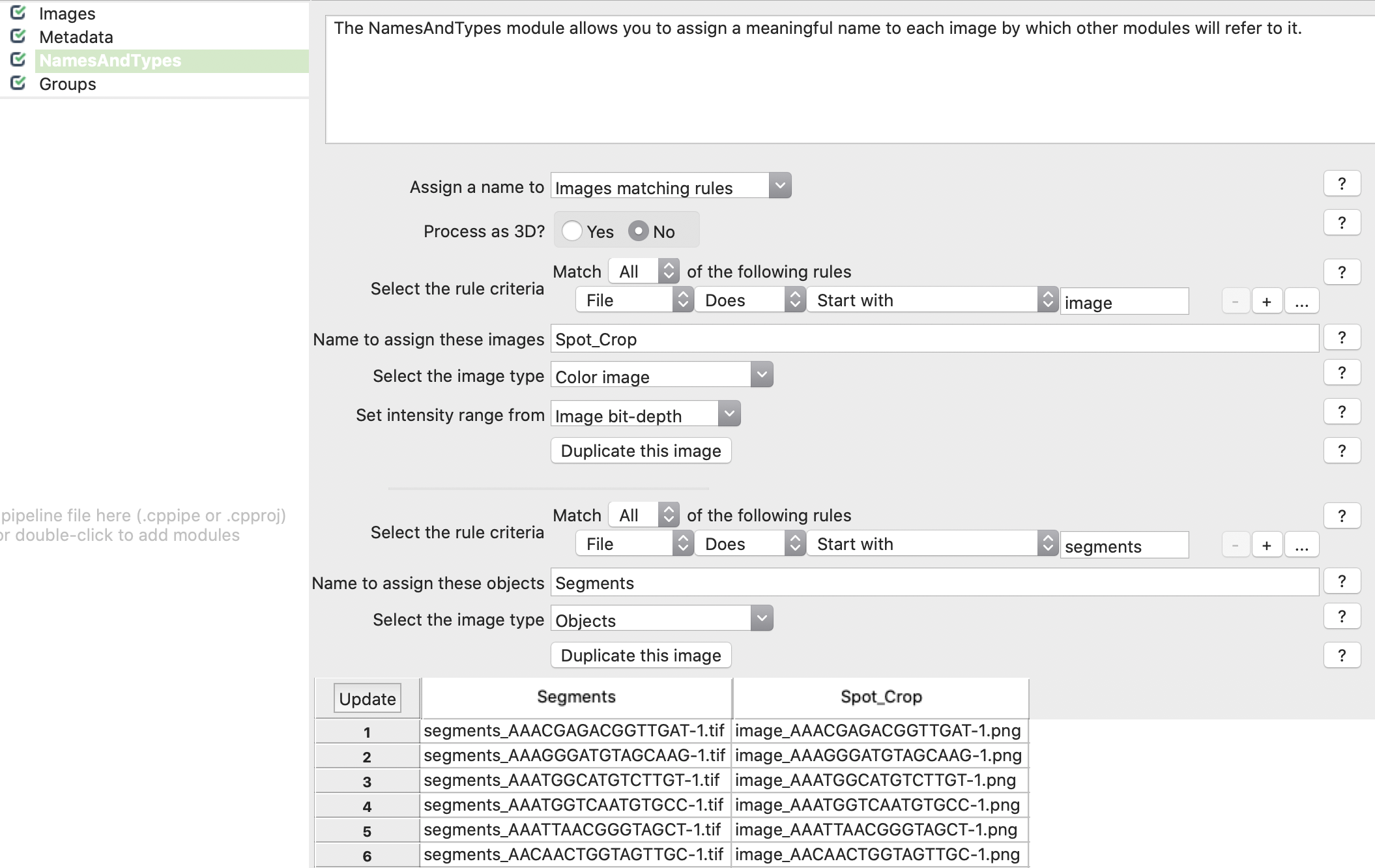
4. CP-Pipeline NamesAndTypes: Declare to load crops as color images and segmentations as objects. Crops and segmentations files are aligned automatically.
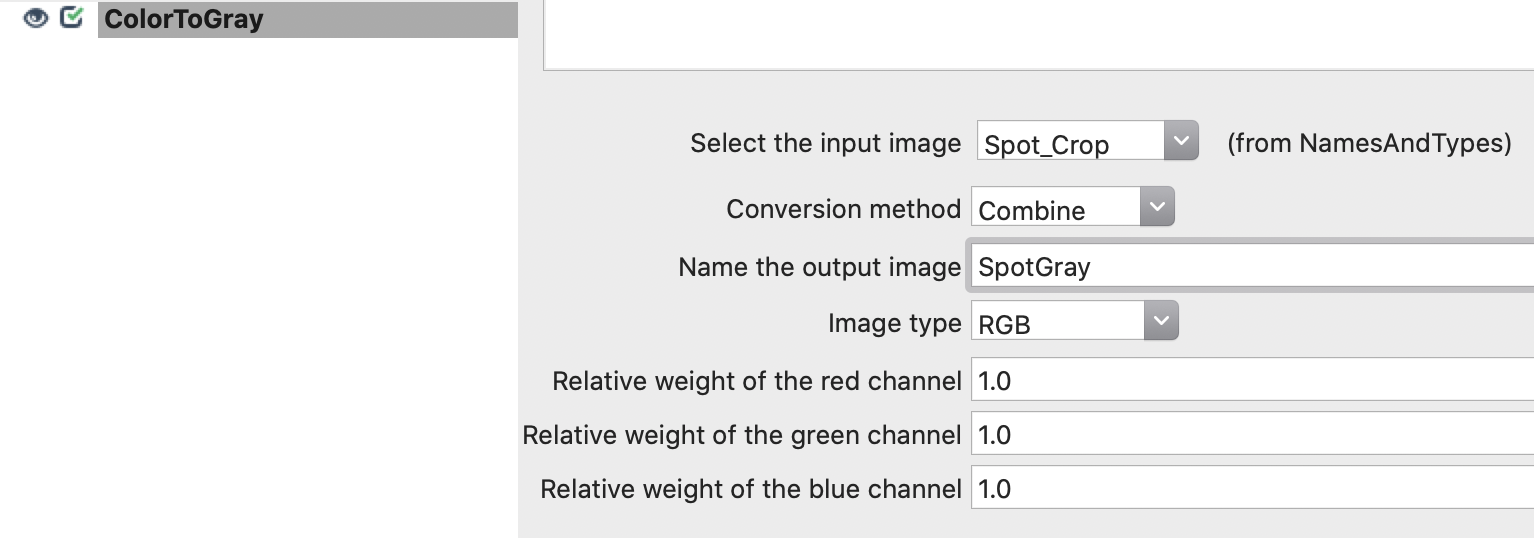
5. Convert image crops to gray images: Add ColorToGray module and define parameters.
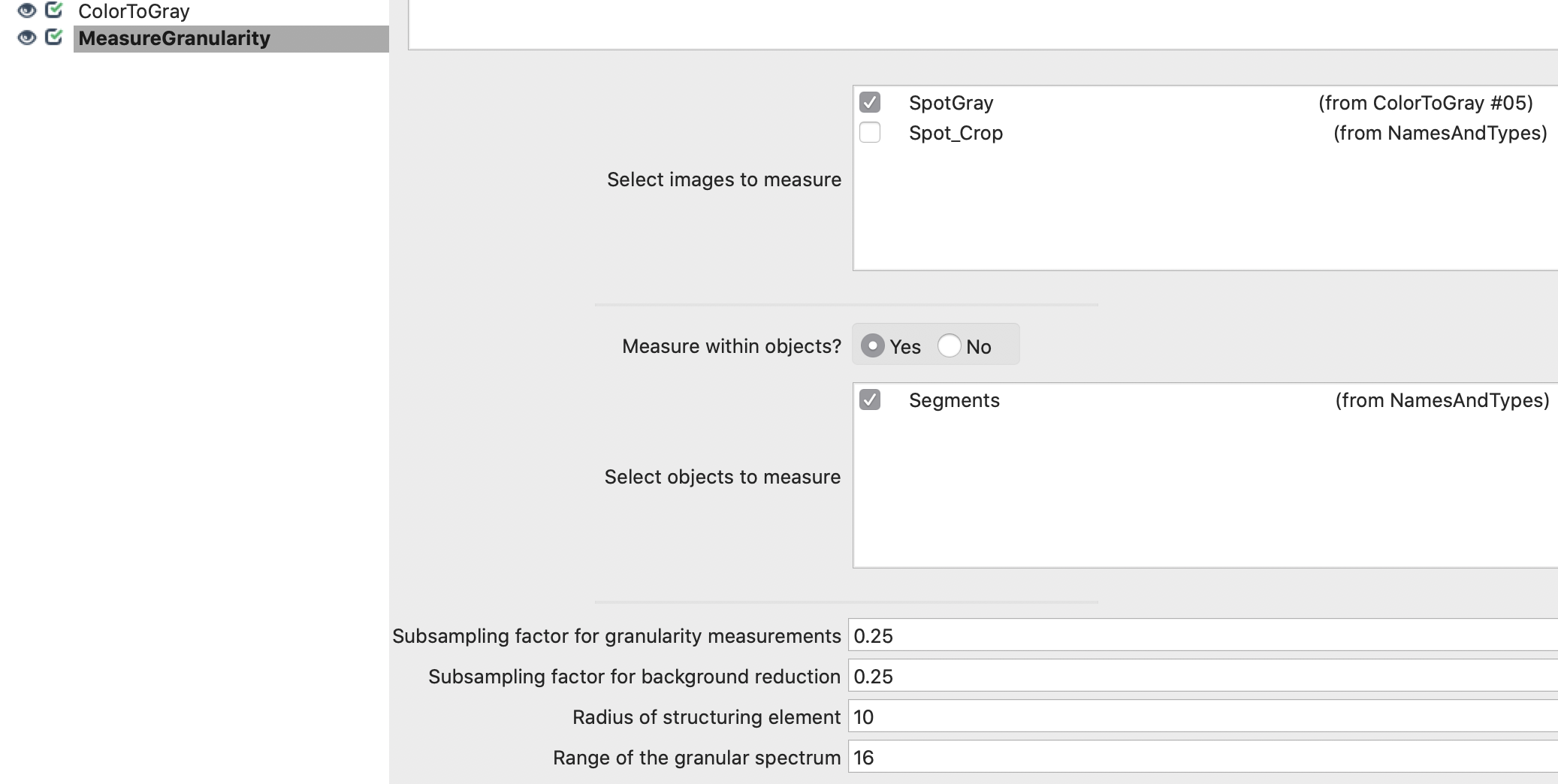
6. Measure CellProfiler’s Granularity features within segments for each crop: Add MeasureGranularity module and define parameters.
7. Export results to csv.
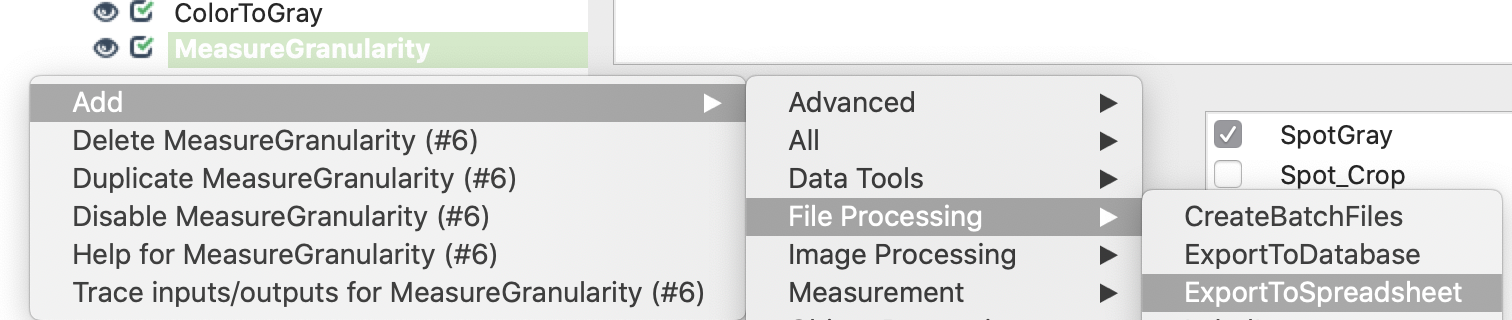
8. Run CellProfiler Pipeline (takes several minutes).
You can now delete the images in imgs_dir by uncommenting the cell below:
# !rm -rf $imgs_dir
Cluster features
Load CellProfiler output of Granularity features of each Visium spot and rearrange the output for adata.obsm.
# load CellProfiler output
df = pd.read_csv(BASE_DIR + "/squidpy_Image.csv")
# set obs names as index
df.index = df["FileName_Spot_Crop"].apply(lambda s: s.split("_")[1].split(".")[0])
df.index.name = "obs"
# Get the measured Granularity features and rename them
features = [
f"{stat}_Segments_Granularity_{i}_SpotGray"
for stat in ["Mean", "StDev"]
for i in range(1, 17)
]
df = df[features]
df.columns = [s.split("_")[0] + s.split("_")[2] + s.split("_")[3] for s in df.columns]
df.head()
| MeanGranularity1 | MeanGranularity2 | MeanGranularity3 | MeanGranularity4 | MeanGranularity5 | MeanGranularity6 | MeanGranularity7 | MeanGranularity8 | MeanGranularity9 | MeanGranularity10 | ... | StDevGranularity7 | StDevGranularity8 | StDevGranularity9 | StDevGranularity10 | StDevGranularity11 | StDevGranularity12 | StDevGranularity13 | StDevGranularity14 | StDevGranularity15 | StDevGranularity16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| obs | |||||||||||||||||||||
| AAACGAGACGGTTGAT-1 | 35.513010 | 8.562676 | 26.546550 | 14.187602 | 7.629247 | 3.334912 | 2.057867 | 0.623025 | 0.258596 | 0.336359 | ... | 1.615079 | 0.490024 | 0.284772 | 0.251887 | 0.000000 | 0.000000 | 0.000000 | 0.196002 | 0.000000 | 0.0 |
| AAAGGGATGTAGCAAG-1 | 34.115594 | 24.218906 | 20.290728 | 4.457829 | 0.832083 | 4.670038 | 3.027418 | 1.872175 | 0.694657 | 0.642282 | ... | 4.840615 | 2.934531 | 1.024722 | 1.045698 | 0.174283 | 0.783332 | 0.156181 | 0.156181 | 0.156181 | 0.0 |
| AAATGGCATGTCTTGT-1 | 35.680015 | 15.708321 | 15.551889 | 8.760072 | 5.392920 | 7.575340 | 5.474114 | 0.876559 | 0.121634 | 0.000000 | ... | 2.082841 | 1.640197 | 0.227598 | 0.000000 | 0.392862 | 0.785725 | 1.172769 | 0.234967 | 0.000000 | 0.0 |
| AAATGGTCAATGTGCC-1 | 27.243626 | 6.490712 | 9.602902 | 3.852946 | 3.829664 | 0.000000 | 0.000000 | 7.289044 | 8.814914 | 12.036803 | ... | 0.000000 | 9.020724 | 6.604276 | 3.711367 | 1.988622 | 1.063700 | 0.303914 | 0.075979 | 0.151957 | 0.0 |
| AAATTAACGGGTAGCT-1 | 33.575662 | 12.159623 | 21.226989 | 19.807115 | 8.313644 | 2.021116 | 0.463706 | 0.716149 | 0.283679 | 0.283679 | ... | 0.328582 | 0.264752 | 0.219188 | 0.219188 | 0.000000 | 0.000000 | 0.219188 | 0.000000 | 0.000000 | 0.0 |
5 rows × 32 columns
Load image features into adata.obsm["features"] and compute Leiden clustering.
def cluster_features(features: pd.DataFrame):
"""Calculate leiden clustering of features."""
# create temporary adata to calculate the clustering
adata = ad.AnnData(features)
# important - feature values are not scaled, so need to scale them before PCA
sc.pp.scale(adata)
# calculate leiden clustering
sc.pp.pca(adata, n_comps=min(10, features.shape[1] - 1))
sc.pp.neighbors(adata)
sc.tl.leiden(adata)
return adata.obs["leiden"]
# Save feature in adata.obsm
adata.obsm["features"] = df.loc[adata.obs_names].copy()
# Calculate leiden clustering
adata.obs["features_granularity_cluster"] = cluster_features(adata.obsm["features"])
# Plot clusterings
sq.pl.spatial_scatter(
adata,
color=[
"features_granularity_cluster",
"cluster",
],
ncols=2,
)
/var/folders/pz/fw_kwtn10t52fps52c0jxm940000gn/T/ipykernel_8107/2650860513.py:5: FutureWarning: X.dtype being converted to np.float32 from float64. In the next version of anndata (0.9) conversion will not be automatic. Pass dtype explicitly to avoid this warning. Pass `AnnData(X, dtype=X.dtype, ...)` to get the future behavour.
adata = ad.AnnData(features)
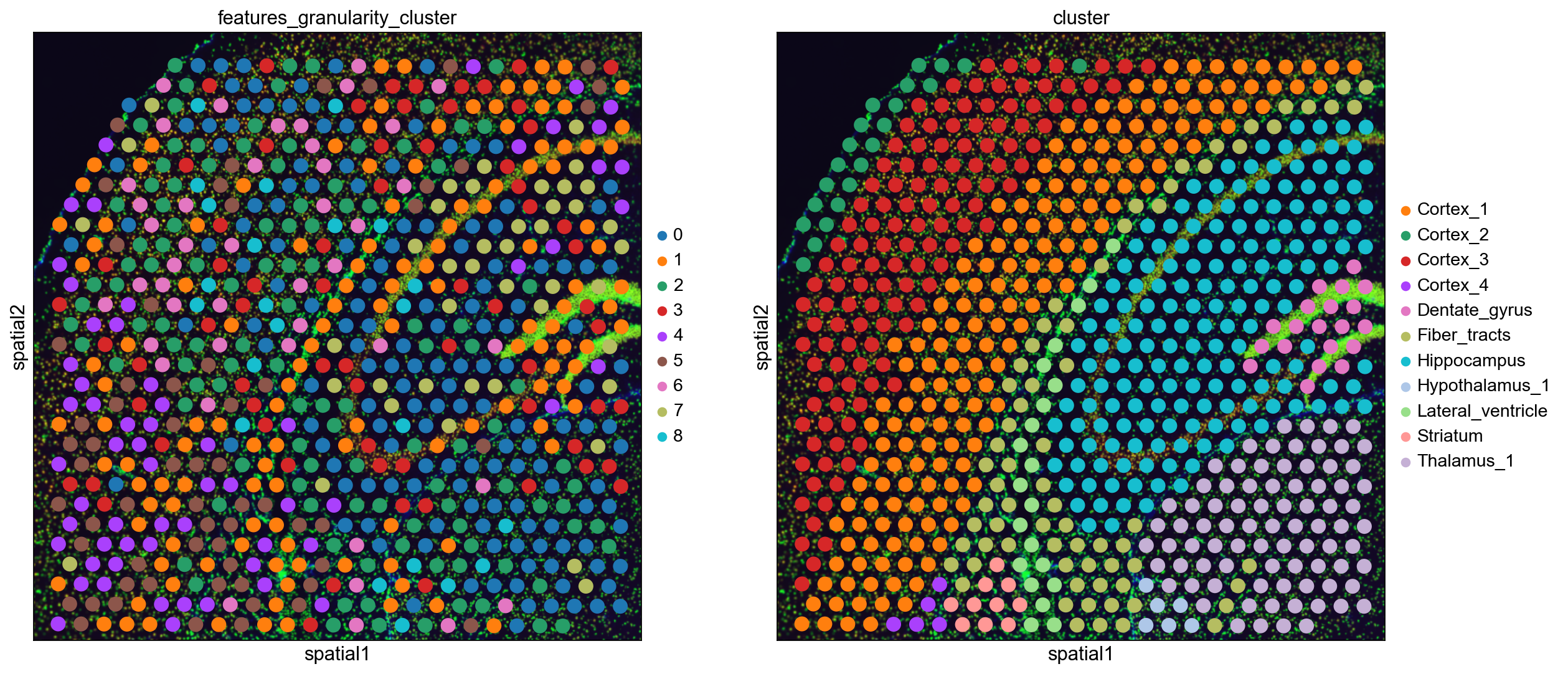
We see that e.g. the blue cluster is prominent in the Hippocampus and the Thalamus, and the brown cluster is prominent in dense regions like Dentate gyrus.
This tutorial is an example on how to integrate CellProfiler within Squidpy pipelines. You can adapt this scheme to other task distributions: e.g. segmentation with CellProfiler and image feature calculation in Squidpy, or process images with CellProfiler, followed by a Squidpy pipeline etc.